
Data Sovereignty for Pharma and Life Sciences Businesses
In today’s digital age, data is the lifeblood of businesses across various industries. This is especially true for the pharma and life sciences sector, where data plays a crucial role in research, development, and regulatory compliance. However, the sensitive nature of this data and the complex global regulations surrounding it make data sovereignty a critical consideration for pharma and life sciences businesses.
What Data Compliance Standards Matter?
Understanding Data Sovereignty
Data sovereignty refers to the concept that data is subject to the laws and regulations of the country in which it is located. It encompasses the control and ownership of data, ensuring that businesses have the authority to dictate how their data is stored, processed, and transferred.
When it comes to data sovereignty, there are several key factors to consider. One of the most important aspects is the protection of valuable and sensitive information. By ensuring that data remains within the borders of a specific country or region, businesses can protect themselves from potential risks such as unauthorized access, data breaches, and legal issues.
For pharma and life sciences businesses, data sovereignty is of paramount importance. These industries deal with highly confidential and sensitive data, including patient records, clinical trial results, and proprietary research. Maintaining control over this data is crucial not only for protecting patients’ privacy but also for complying with strict regulatory requirements.
Definition and Importance of Data Sovereignty
At its core, data sovereignty is about maintaining control over valuable and sensitive information. By ensuring that data remains within the borders of a specific country or region, businesses can protect themselves from potential risks such as unauthorized access, data breaches, and legal issues.
Data sovereignty is not just a matter of convenience; it is a legal and regulatory requirement in many jurisdictions. In some countries, data protection laws explicitly require that certain types of data must be stored and processed within the country’s borders. Failure to comply with these laws can result in severe penalties and reputational damage for businesses.
Furthermore, data sovereignty plays a crucial role in ensuring data integrity and security. By keeping data within a specific jurisdiction, businesses can implement robust security measures tailored to the local regulatory environment. This includes encryption, access controls, and monitoring systems that align with the specific requirements of the country in question.
Data Sovereignty and Global Regulations
Pharma and life sciences businesses operate in a global landscape, making compliance with international regulations a complex task. Different countries have varying data protection laws, such as the European Union’s General Data Protection Regulation (GDPR), the Health Insurance Portability and Accountability Act (HIPAA) in the United States, and the Personal Information Protection and Electronic Documents Act (PIPEDA) in Canada.
Adhering to these regulations requires businesses to understand the requirements of each jurisdiction where they operate and ensure that data is stored and processed in compliance with the applicable laws. This is where data sovereignty becomes crucial, as it enables businesses to maintain control over their data while meeting regulatory requirements.
For example, the GDPR imposes strict rules on the transfer of personal data outside the European Economic Area (EEA). To comply with these regulations, businesses must ensure that data is stored and processed within the EEA or in countries that have been deemed to provide an adequate level of data protection. This requirement highlights the importance of data sovereignty in enabling businesses to navigate complex international regulations.
In addition to legal compliance, data sovereignty also has implications for business continuity and disaster recovery. By keeping data within a specific jurisdiction, businesses can ensure that they have access to their critical data even in the event of a natural disaster or other disruptive events. This level of control and accessibility is essential for maintaining operations and minimizing downtime.
In conclusion, data sovereignty is a critical consideration for businesses, particularly in industries dealing with sensitive information. It enables businesses to maintain control over their data, comply with international regulations, and ensure data integrity and security. By understanding the importance of data sovereignty and implementing appropriate measures, businesses can protect themselves and their stakeholders from potential risks and legal issues.
Data Sovereignty in Pharma and Life Sciences
Data sovereignty has unique implications for the pharma and life sciences industry. These sectors heavily rely on data for research, clinical trials, drug development, and post-marketing surveillance. Let’s explore the role of data sovereignty in each of these areas.
Role of Data Sovereignty in Pharma
In the pharmaceutical sector, data sovereignty is essential for protecting valuable intellectual property (IP). The industry invests significant resources and time in research and development, making it crucial to safeguard proprietary data. Data sovereignty enables pharma companies to control access to their IP, preventing unauthorized use and theft.
Moreover, data sovereignty plays a critical role in ensuring data integrity and confidentiality. Pharmaceutical companies deal with sensitive patient information, clinical trial data, and proprietary research findings. By implementing robust data sovereignty measures, these companies can protect the privacy and confidentiality of individuals involved in clinical trials and research studies.
Data sovereignty also aids in maintaining regulatory compliance. Pharmaceutical companies must adhere to rigorous regulations imposed by government bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in the European Union. By ensuring data sovereignty, companies can demonstrate compliance and streamline the regulatory approval processes.
Furthermore, data sovereignty empowers pharma companies to leverage their data for competitive advantage. By having control over their data, these companies can analyze it to gain insights, identify patterns, and make data-driven decisions. This can lead to improved drug development processes, personalized medicine approaches, and enhanced patient outcomes.
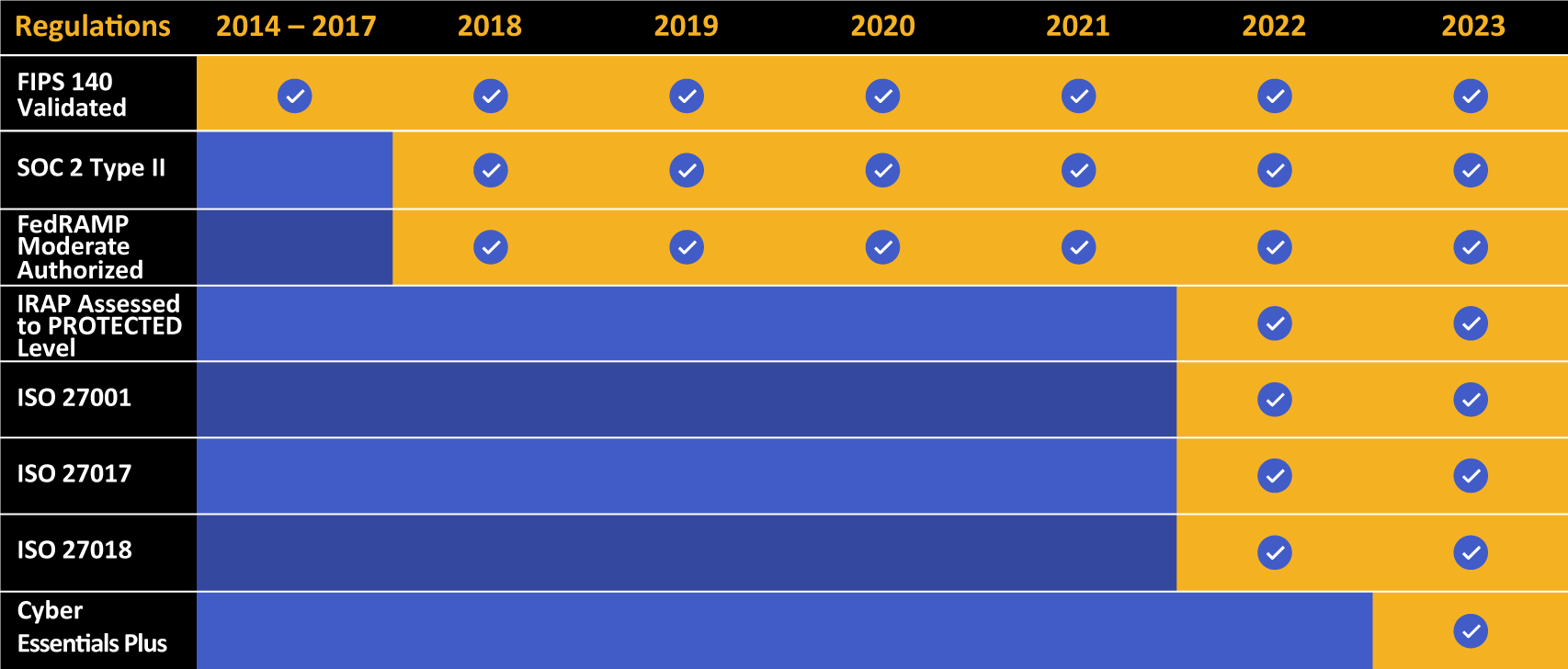
Kiteworks touts a long list of compliance and certification achievements.
Impact of Data Sovereignty on Life Sciences
Life sciences encompass a broad range of scientific disciplines, including biotechnology, genetics, and bioinformatics. These fields generate vast amounts of data, from genomics data to clinical trial results. Protecting and governing this data is crucial for advancing scientific research and promoting discoveries that can improve human health.
Data sovereignty facilitates collaboration within the life sciences industry while ensuring data protection. By maintaining control over data, researchers and institutions can share information securely, adhere to data usage agreements, and protect against unauthorized sharing or misuse of sensitive data.
In addition, data sovereignty enables efficient data sharing and integration across different research organizations and institutions. This promotes interdisciplinary collaborations and accelerates scientific discoveries. For example, researchers studying the genetic basis of diseases can securely access and analyze genomic data from various sources, leading to a deeper understanding of complex diseases and the development of targeted therapies.
Data sovereignty also plays a crucial role in ensuring reproducibility and transparency in life sciences research. By having control over their data, researchers can provide access to their datasets, methodologies, and analysis techniques, allowing other scientists to validate and build upon their findings. This fosters a culture of open science and drives innovation in the life sciences field.
Furthermore, data sovereignty enables the responsible and ethical use of data in life sciences. Researchers can implement data governance frameworks to ensure compliance with ethical guidelines, privacy regulations, and data protection laws. This helps to build trust among stakeholders, including patients, research participants, and regulatory bodies.
As the pharma and life sciences industries continue to generate and rely on vast amounts of data, data sovereignty will remain a critical consideration. By understanding its role and impact, stakeholders can navigate the complex landscape of data management, privacy, and security, ultimately driving innovation and improving patient outcomes.
Challenges in Implementing Data Sovereignty
Implementing data sovereignty in pharma and life sciences businesses comes with its own set of challenges. Let’s examine the main hurdles faced in achieving data sovereignty.
Legal and Regulatory Challenges
A major challenge in implementing data sovereignty is navigating the complex web of legal and regulatory frameworks. As mentioned earlier, different countries have different data protection laws, and businesses must ensure compliance with each jurisdiction’s requirements. This can involve legal consultations, adopting standardized practices, and implementing robust data protection measures to meet the stringent demands.
Furthermore, data sovereignty also raises questions about cross-border data transfers. When data needs to be transferred from one country to another, businesses must ensure that the data remains protected and compliant with both the source and destination country’s regulations. This can involve negotiating data transfer agreements, implementing data localization measures, or utilizing secure data transfer protocols.
Moreover, data sovereignty can also clash with international data sharing initiatives. In the field of pharma and life sciences, collaboration and data sharing are crucial for research and development. However, data sovereignty can create barriers to such collaborations, as businesses may be hesitant to share sensitive data due to concerns about data protection and compliance.
Technological Challenges
From a technological standpoint, implementing data sovereignty requires robust infrastructure and data management systems. Businesses need to invest in secure storage facilities, backup systems, encryption technologies, and access controls to protect data from unauthorized access or breaches. Additionally, companies must ensure data integrity and availability while complying with evolving technological standards and protocols.
Implementing data sovereignty also requires businesses to address the challenges of data interoperability and integration. As data is stored and managed across different systems and platforms, ensuring seamless data exchange and integration becomes crucial. This may involve implementing data governance frameworks, adopting standardized data formats, and establishing secure APIs for data sharing.
Furthermore, the increasing volume and complexity of data in pharma and life sciences pose additional technological challenges. Businesses must invest in advanced analytics and data processing capabilities to effectively manage and derive insights from large datasets. This can involve deploying machine learning algorithms, implementing data lakes or data warehouses, and utilizing cloud computing technologies for scalable data processing.
Lastly, ensuring data sovereignty also requires businesses to stay updated with emerging technologies and trends. As technology evolves, new security threats and vulnerabilities may arise, requiring continuous monitoring and adaptation of data protection measures. This can involve regular security audits, penetration testing, and staying informed about the latest advancements in cybersecurity.
Strategies for Achieving Data Sovereignty
While data sovereignty may seem like a daunting task, there are strategies that pharma and life sciences businesses can employ to achieve and maintain control over their data.
Adopting a Data-Centric Approach
One effective strategy is adopting a data-centric approach to data management. This involves understanding the criticality of data, its lifecycle, and implementing appropriate security and governance measures at each stage. By viewing data as a valuable asset and implementing data-centric practices, businesses can enhance data sovereignty.
Leveraging Cloud Technology for Data Sovereignty
Cloud technology offers significant advantages for achieving data sovereignty. Cloud service providers can facilitate data storage and processing within specified regions or countries, ensuring compliance with local data protection regulations. By partnering with cloud providers that prioritize data sovereignty and offer robust security measures, pharma and life sciences businesses can leverage the benefits of the cloud while maintaining control over their data.
Future of Data Sovereignty in Pharma and Life Sciences
As technology continues to evolve and regulatory landscapes change, the future of data sovereignty in the pharma and life sciences industry holds several promising trends and developments.
Predicted Trends and Developments
One notable trend is the increasing adoption of blockchain technology in securing data sovereignty. Blockchain offers enhanced data integrity, transparency, and traceability, making it an ideal solution for ensuring the authenticity and protection of sensitive data.
Another development is the growth of data protection legislation worldwide. Governments are recognizing the importance of data sovereignty and passing regulations to safeguard the personal information of citizens. This shift towards stricter data protection measures further highlights the significance of data sovereignty in the pharma and life sciences sectors.
Preparing for the Future of Data Sovereignty
Pharma and life sciences businesses must stay agile and proactive to navigate the future of data sovereignty successfully. This entails staying abreast of changing regulations, investing in robust data protection technologies, and fostering a culture of data governance within the organization.
Kiteworks Helps Pharma and Life Sciences Businesses Comply with Data Sovereignty Requirements
By prioritizing data sovereignty and taking proactive measures, pharma and life sciences businesses can navigate the complex landscape of data management, protect their valuable assets, and ensure compliance with global regulations.
The Kiteworks Private Content Network, a FIPS 140-2 Level 1 validated secure file sharing and file transfer platform, consolidates email, file sharing, web forms, SFTP and managed file transfer, so organizations control, protect, and track every file as it enters and exits the organization.
Kiteworks plays a crucial role in pharma and life sciences businesses’ data sovereignty efforts. For example, Kiteworks’ encryption and access control features protect personal information during cross-border transfers, ensuring secure transmission.
Kiteworks’ extensive deployment options, including private, hybrid, and FedRAMP virtual private cloud, can be configured to store data in specific geographic locations. By storing data in specific locations, organizations can ensure that they are adhering to the data sovereignty laws of the countries in which they operate.
Kiteworks also supports data portability requirements by enabling users to securely access, transfer, and download their personal information. Kiteworks also provides organizations with the ability to establish opt-in mechanisms and procedures for data collection, detailed consent forms, and minor consent procedures. These features help organizations comply with consent requirements, which are a key aspect of data sovereignty.
Finally, Kiteworks’ detailed audit trail enables organizations to prove their compliance with data sovereignty laws to auditors.
With Kiteworks: control access to sensitive content; protect it when it’s shared externally using automated end-to-end encryption, multi-factor authentication, and security infrastructure integrations; see, track, and report all file activity, namely who sends what to whom, when, and how.
Finally demonstrate compliance with regulations and standards like GDPR, HIPAA, CMMC, Cyber Essentials Plus, IRAP, and many more.
To learn more about Kiteworks, schedule a custom demo today.
Additional Resources
- Brief Expand Visibility and Automate Protection of All Sensitive Email
- Brief Navigate the Digital Trifecta of Data Sovereignty, Cybersecurity, and Compliance With Kiteworks
- Blog Post Data Sovereignty and GDPR [Understanding Data Security]
- Video What Is Email Security? How to Protect Your Sensitive Content With Email Security
- Brief Secure Protocol Package: Strengthening Data Exchange With SFTP and SMTP